Protein tyrosine phosphatases
The kinases have been implicated in controlling the amplitude of a signalling response, whereas phosphatases are thought to have an important role in controlling the rate and duration of the response [1, 2].
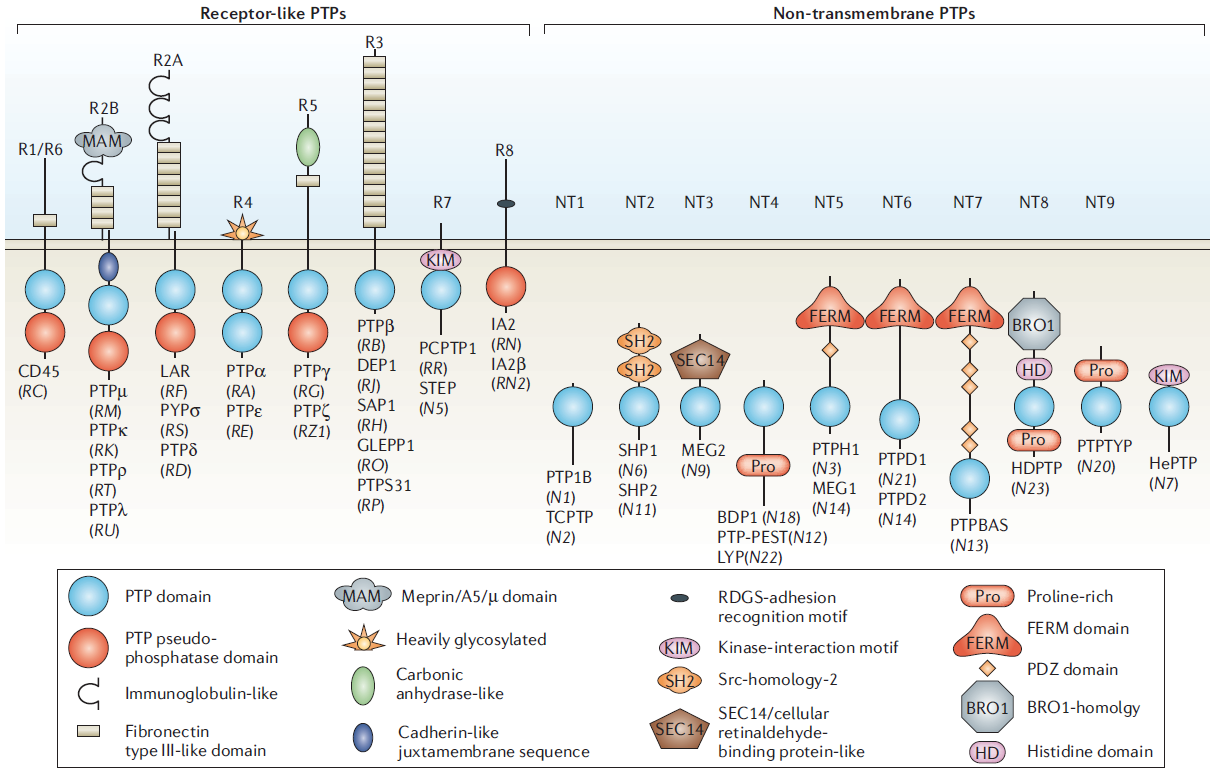
The classical PTPs. The classical protein tyrosine phosphatases (PTPs) can be categorized as receptor-like (R) or non-transmembrane (NT) proteins. However, this is not an absolute distinction. For example, use of alternative promoters (as in the case of PTPRE) or alternative splicing (as in the case of GLEPP1 (PTPRO)) leads to the production of transmembrane and cytoplasmic forms of some PTPs from a single gene. In the PTPR7 subgroup, receptor-like and nontransmembrane variants of PCPTP1 (PTPRR) and STEP (PTPN5) have been described; however, as no receptor-like isoforms of HePTP (PTPN7) have been identified, this is included with the non-transmembrane enzymes. For those receptor-like PTPs (RPTP) with two intracellular PTP domains, the membrane-proximal D1 domain is catalytically active. In receptor subtype R4, PTPalpha is unique among the RPTPs in that the membrane-distal D2 domain also displays a low residual activity. For the remaining RPTPs, including PTPepsilon, which is the other enzyme in receptor subtype R4, the D2 domain maintains a PTP fold but lacks activity and can be classified as a pseudophosphatase domain. In each case, the PTPs have been designated by a name that is commonly used in the literature. Where this differs from the gene symbol, the latter is included in parentheses for clarification. In each case, the various subdivisions are based upon sequence similarity [3].
- (2023) Nature. 622(7984):850-862. doi: 10.1038/s41586-023-06575-7. The PTPN2/PTPN1 inhibitor ABBV-CLS-484 unleashes potent anti-tumour immunity.
- 2023) Nat Commun. 14(1):4524. doi: 10.1038/s41467-023-40170-8. A small molecule inhibitor of PTP1B and PTPN2 enhances T cell anti-tumor immunity.
- (2023) J. Med. Chem. 66, 19, 13384-13399. doi: 10.1021/acs.jmedchem.3c00483. Identification of GDC-1971 (RLY-1971), a SHP2 Inhibitor Designed for the Treatment of Solid Tumors.
- (2023) ACS Med. Chem. Lett. 14, 2, 156-162. doi: 10.1021/acsmedchemlett.2c00454. Discovery of a Novel Series of Imidazopyrazine Derivatives as Potent SHP2 Allosteric Inhibitors.
- (2022) iScience 25: 104009-104009. doi: 10.1016/j.isci.2022.104009. SHP2 allosteric inhibitor TK-453 alleviates psoriasis-like skin inflammation in mice via inhibition of IL-23/Th17 axis.
- (2012) J Am Chem Soc 134: 18116-18124. doi: 10.1021/ja308212y. A Highly Selective and Potent PTP-MEG2 Inhibitor with Therapeutic Potential for Type 2 Diabetes.
- (2013) J Med Chem 56: 4990-5008. doi: 10.1021/jm400248c. A Potent and Selective Small-Molecule Inhibitor for the Lymphoid-Specific Tyrosine Phosphatase (LYP), a Target Associated with Autoimmune Diseases.
- (2010) J. Med. Chem., 53, 6, 2482-2493. doi: 10.1021/jm901645u. Salicylic Acid Based Small Molecule Inhibitor for the Oncogenic Src Homology-2 Domain Containing Protein Tyrosine Phosphatase-2 (SHP2).
- (2022) Mol Oncol., 16(21):3761-3777. doi: 10.1002/1878-0261.13277. Fragment-based drug discovery—the importance ofhigh-quality molecule libraries.
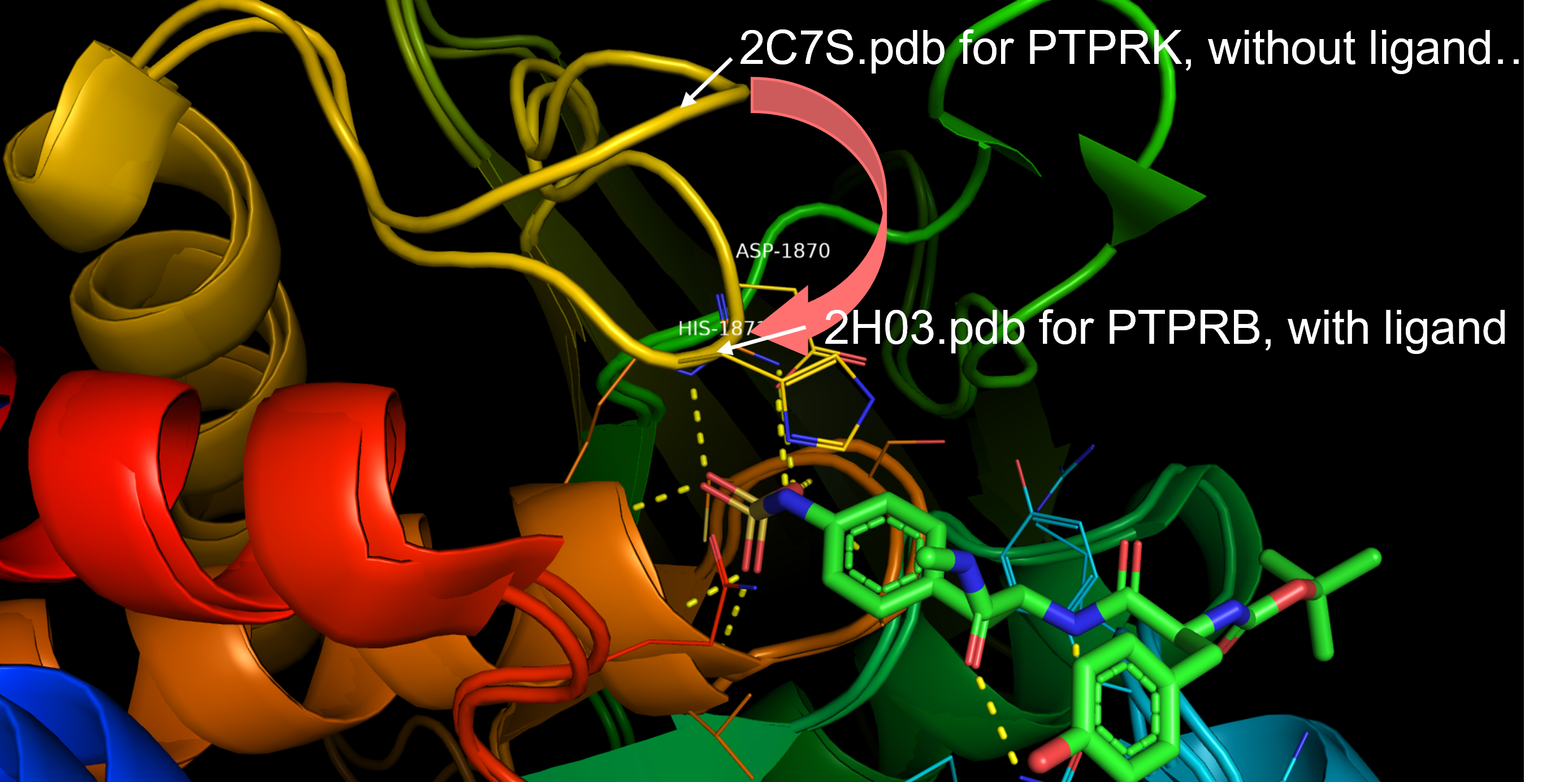
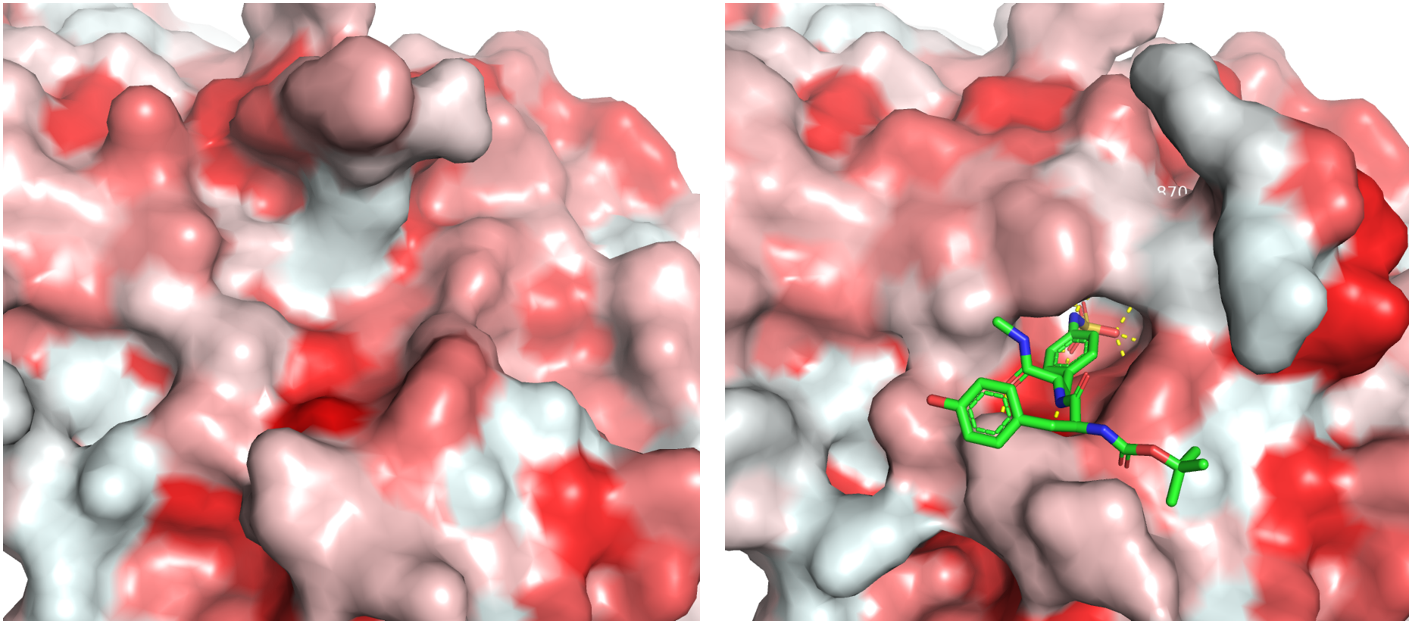
- 1.- PTPRC: P08575 - PTPRC_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 2.- PTPRM: P28827 - PTPRM_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 3.- PTPRK: Q15262 - PTPRK_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 4.- PTPRT: O14522 - PTPRT_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 5.- PTPRU: Q92729 - PTPRU_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 6.- PTPRF: P10586 - PTPRF_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 7.- PTPRS: Q13332 - PTPRS_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 8.- PTPRD: P23468 - PTPRD_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 9.- PTPRA: P18433 - PTPRA_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 10.- PTPRE: P23469 - PTPRE_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 11.- PTPRG: P23470 - PTPRG_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 12.- PTPRZ1: P23471 - PTPRZ_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 13.- PTPRB: P23467 - PTPRZ_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 14.- PTPRJ (HPTPeta, CD148, DEP1): Q12913 - PTPRJ_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 15.- PTPRH (R-PTP-H, SAP1): Q9HD43 - PTPRH_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 16.- PTPRO (GLEPP1, PTP-U2, PTPU2, NPHS6): Q16827 - PTPRO_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 17.- PTPRQ (R-PTP-Q, DFNB84): Q9UMZ3 - PTPRQ_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 18.- PTPRR (PTPBR7, PTP-SL, EC-PTP, PCPTP1, PTPRQ): Q15256 - PTPRR_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 19.- PTPN5 (STEP, PTPSTEP, STEP61): P54829 - PTN5_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 20.- PTPRN: Q16849 - PTPRN_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 21.- PTPRN2: Q92932 - PTPR2_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 22.- PTPN1 (PTP1B): P18031 - PTN1_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 23.- PTPN2 (TCPTP, TCELLPTP, TC-PTP, PTPT): P17706 - PTN2_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 24.- PTPN6 (HCP, PTP1C): P29350 - PTN6_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 25.- PTPN11 (PTP2C, SHPTP2): Q06124 - PTN11_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 26.- PTPN9 (PTPMEG2): P43378 - PTN9_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 27.- PTPN18 (BDP1): Q99952 - PTN18_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 28.- PTPN12 (PTP-PEST, PTPG1): Q05209 - PTN12_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 29.- PTPN22 (PTPN8): Q9Y2R2 - PTN22_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 30.- PTPN3 (PTPH1): P26045 - PTN3_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 31.- PTPN4 (PTPMEG): P29074 - PTN4_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 32.- PTPN21 (PTPD1, PTPRL10): Q16825 - PTN21_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 33.- PTPN14 (PTPD2, PEZ, PTP36, CATLPH): Q15678 - PTN14_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 34.- PTPN13 (PTP-BAS, PTP1E, PTPL1, PTP-BL): Q12923 - PTN13_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 35.- PTPN23 (HD-PTP, KIAA1471): Q9H3S7 - PTN23_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 36.- PTPN20 (BA142I17.1, BA42B19.1, PTPN20A, PTPN20B, CT126): Q4JDL3 - PTN20_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 37.- PTPN7 (BPTP-4, PTPNI, LPTP): P35236 - PTN7_HUMAN. RCBS link to PDBs. Full-sequence. GeneCards.
- 1.- PTPRC, P08575 - PTPRC_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 2.- PTPRM, P28827 - PTPRM_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 3.- PTPRK, Q15262 - PTPRK_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 4.- PTPRT, O14522 - PTPRT_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 5.- PTPRU, Q92729 - PTPRU_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 6.- PTPRF, P10586 - PTPRF_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 7.- PTPRS, Q13332 - PTPRS_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 8.- PTPRD, P23468 - PTPRD_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 9.- PTPRA, P18433 - PTPRA_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 10.- PTPRE, P23469 - PTPRE_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 11.- PTPRG, P23470 - PTPRG_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 12.- PTPRZ1, P23471 - PTPRZ_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 13.- PTPRB, P23467 - PTPRB_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 14.- PTPRJ, Q12913 - PTPRJ_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 15.- PTPRH, Q9HD43 - PTPRH_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 16.- PTPRO, Q16827 - PTPRO_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 17.- PTPRQ, Q9UMZ3 - PTPRQ_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 18.- PTPRR, Q15256 - PTPRR_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 19.- PTPN5, P54829 - PTN5_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 20.- PTPRN, Q16849 - PTPRN_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 21.- PTPRN2, Q92932 - PTPR2_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 22.- PTPN1, P18031 - PTN1_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 23.- PTPN2, P17706 - PTN2_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 24.- PTPN6, P29350 - PTN6_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 25.- PTPN11, Q06124 - PTN11_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 26.- PTPN9, P43378 - PTN9_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 27.- PTPN18, Q99952 - PTN18_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 28.- PTPN12, Q05209 - PTN12_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 29.- PTPN22, Q9Y2R2 - PTN22_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 30.- PTPN3, P26045 - PTN3_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 31.- PTPN4, P29074 - PTN4_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 32.- PTPN21, Q16825 - PTN21_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 33.- PTPN14, Q15678 - PTN14_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 34.- PTPN13, Q12923 - PTN13_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 35.- PTPN23, Q9H3S7 - PTN23_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 36.- PTPN20, Q4JDL3 - PTN20_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL. - 37.- PTPN7, P35236 - PTN7_HUMAN. FASTA and D1-full protein sequence alignment.
 Closed D1 domain SWISS-MODEL.
Closed D1 domain SWISS-MODEL.