The free energy change for the chemical reaction
A + B → C + D
can be expressed in terms of a constant characteristic for that reaction (the standard free energy change, ΔGo') and the concentrations of reactants and products (the mass action ratio Q =[C][D]/[A][B]). Equation:
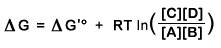
For the reaction catalyzed by hexokinase
ATP + glucose → glucose 6-phosphate + ADP.
the standard free energy change ΔGo' is -16.7 kJ/mol. Use the living graph to plot ΔG' vs. the mass action ratio Q =[C][D]/[A][B], over the range 10-4 to 104 for Q. What can you conclude about the effect of Q on the magnitude of ΔG'? About the effect of Q on the sign of ΔG'?
To use the graph enter the parameters and hit New Plot. Up to 5 plots can be displayed at one time. The Clear button will remove all plots. To see the parameters for each plot hit the Legend button. The Redraw button will refresh the graph. This is useful when the function domain (i.e., [C][D] / [A][B]) has been changed. To see the value of each plot at a given point, move your cursor to the desired location then click and hold.
Original material from Lehninger Principles of Biochemistry, 5a edición, de D. Nelson and M. Cox; 2009. ISBN: 0-7167-7108-X.
Dr. José Antonio Encinar. (IBMC-UMH)