Proteins function by interacting with other molecules. Molecules that interact reversibly with proteins, without being altered by the interaction, are called ligands. Protein-ligand interactions are at the heart of countless biochemical processes. For this reason, understanding how these interactions are studied and described quantitatively is fundamental to Biochemistry. As described in the text, the key parameter used to describe these interactions is the dissociation constant, Kd. The exercise below will help you understand how the Kd relates the ligand concentration to the fraction of protein binding sites that are occupied by ligand (a parameter called θ). A protein with a high affinity for its ligand will have a low Kd, meaning it will bind to the ligand at quite low ligand concentrations. Similarly, a protein with a low affinity for its ligand will have a high Kd.
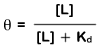
Use the default values and have the computer draw a ligand binding curve by clicking on New Plot. Then decrease the Kd by a factor of 10, and draw a new plot. Note how the curve moves to the left as the Kd decreases. Decrease the Kd by another factor of 10, and draw a new plot again. Insert your own values of Kd, and the range over which the X axis will plot the parameter θ, until you have familiarized yourself with how the equation works.
There is an important point on each of these curves. Note that when the [L] = Kd, the θ = 0.5. This is another way of saying that the Kd (which has units of molarity) is equivalent to the ligand concentration at which the protein binding sites are half saturated with bound ligand.
Student hints: When the Kd increases (weaker binding), the curve shifts to the right. When the Kd decreases (tighter binding), the curve shifts to the left.
Up to 5 plots can be displayed at one time. The Clear button will remove all plots. To see Kd for each plot hit the Legend button. The Redraw button will refresh the graph. This is useful when the function domain (i.e., [L]) has been changed. To see the value of each plot at a given point, move your cursor to the desired location then click and hold.
Original material from Lehninger Principles of Biochemistry, 5a edición, de D. Nelson and M. Cox; 2009. ISBN: 0-7167-7108-X.
Dr. José Antonio Encinar. (IBMC-UMH)