The Michaelis-Menten equation (below) describes quantitatively the relation ship between the rate of an enzyme-catalyzed reaction and the concentration of substrate present, given the parameters Vmax and Km for that enzyme. In this exercise, start with the default values, and create a plot by clicking on New Plot. You will generate a hyperbolic curve describing the activity of a hypothetical enzyme. Determine how the shape of the curve changes if you increase or decrease Km by a factor of 2. Determine how the curve changes when you increase Vmax by a factor of 2. Manipulate the values for the different parameters until you are comfortable with how this equation works.
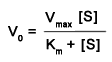
Student hints: The Km in the Michaelis-Menten equation has a role in the graph similar to that of Kd in equation 5.8. A lower Km indicates that the enzyme functions better at lower substrate concentrations. At V = 1/2 Vmax, the substrate concentration is equal to the Km.
Up to 5 plots can be displayed at one time. The Clear button will remove all plots. To see Vmax and Km for each plot hit the Legend button. The Redraw button will refresh the graph. This is useful when the function domain (i.e., [S]) has been changed. To see the value of each plot at a given point, move your cursor to the desired location then click and hold.
Original material from Lehninger Principles of Biochemistry, 5a edición, de D. Nelson and M. Cox; 2009. ISBN: 0-7167-7108-X.
Dr. José Antonio Encinar. (IBMC-UMH)